54. Identification of Active Sites Formed on Cobalt Oxyhydroxide in Glucose Electrooxidation
Zhu, Y. Q.,Zhou, H.*,Dong, J.,Xu, S. M.,Xu, M.,Zheng, L.,Xu, Q.,Ma, L.,Li, Z.,Shao, M.,Duan, H.*
Angew. Chem. Int. Ed. 2023, 62 (15), e202219048.
DOI: 10.1002/anie.202219048

Abstract
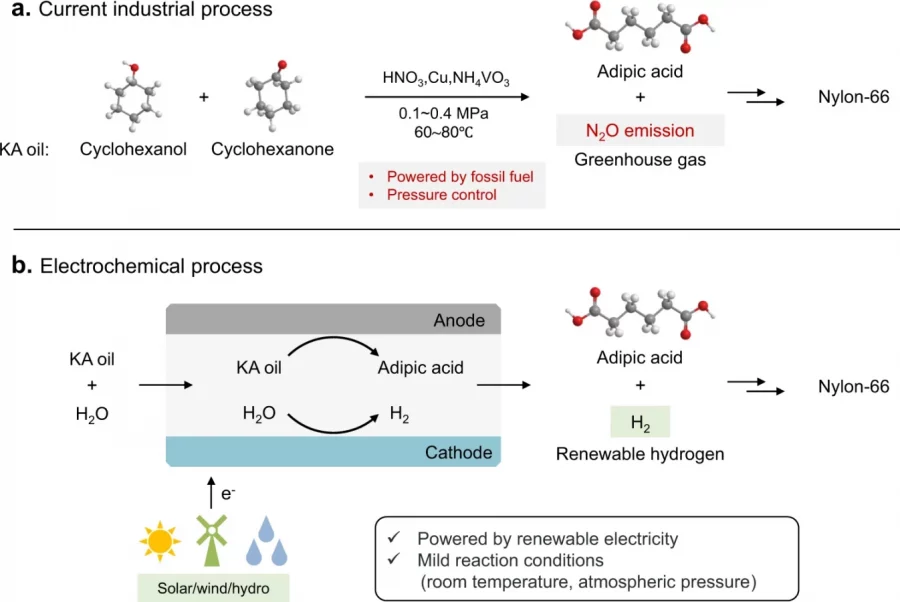
Transition-metal-based oxyhydroxides are efficient catalysts in biomass electrooxidation towards fossil fuel-free production of valuable chemicals. However, identification of active sites remains elusive. Herein, using cobalt oxyhydroxide (CoOOH) as the archetype and electrooxidation of glucose (GOR) as the model reaction, we track dynamic transformation of electronic and atomic structure of the catalyst using a suite of operando and ex situ techniques. We reveal that two types of reducible Co3+−oxo species are affordable for GOR, including adsorbed hydroxyl on Co3+ ion (µ1−OH−Co3+) and di-Co3+-bridged lattice oxygen (µ2−O−Co3+). Moreover, theoretical calculations unveil that µ1−OH−Co3+ is responsible for oxygenation, while µ2−O−Co3+ mainly contributes to dehydrogenation, both as key oxidative steps in glucose-to-formate transformation. This work provides a framework for mechanistic understanding of the complex near-surface chemistry of metal oxyhydroxides in biomass electrorefining.