88. Sustainable oxime production via the electrosynthesis of hydroxylamine in a free state
Jing Li, Xiang Liu, Si-Min Xu, Ming Xu, Yunlong Wang, Yizheng Lyu, An-Zhen Li, Ye Wang, Xi Wang, Tiancong Zhou, Hua Zhou, Yue Peng, Xuning Li, Lirong Zheng & Haohong Duan*
Nature Synthesis, 2025, 4, 1598–1609
DOI: 10.1038/s44160-025-00879-4
Abstract
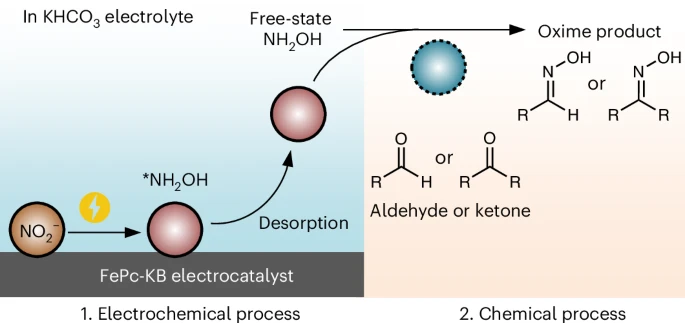
Hydroxylamine (NH2OH) is an important feedstock for oxime production. Coreduction of NOx and aldehydes or ketones enables sustainable one-step oximation by utilizing in situ *NH2OH intermediates but suffers from side reactions and reduced current density due to the presence of multiple reactants in one reactor. Here we decouple oximation into two steps, the electrochemical synthesis of free NH2OH via nitrite (NO2−) electroreduction and the aldehyde or ketone oximation chemical step, circumventing the negative effects (such as site blocking, aldehyde or ketone electroreduction, or crossover) encountered in one-step oximation. By using a Ketjen-black-supported iron phthalocyanine as the catalyst, we achieve an exceptionally high partial current density of free NH2OH (jNH2OH) of 262.9 mA cm−2 (corresponding to productivity of 2.452 mmol cm−2 h−1) in neutral conditions at an industrially relevant current density of 500 mA cm−2. By coupling NH2OH electrosynthesis with subsequent oximation in two steps, nearly stoichiometric oximes are produced with high efficiency and broad applicability. This work paves the way toward a sustainable oxime industry.