43. Engineering Hydrogen Generation Sites to Promote Electrocatalytic CO2 Reduction to Formate
Guo, X., Xu, S.-M., Zhou, H., Ren, Y., Ge, R., Xu, M., Zheng, L., Kong, X., Shao, M., Li, Z.*, Duan, H.*
ACS Catal. 2022, 10551-10559.
DOI: 10.1021/acscatal.2c02548
Abstract
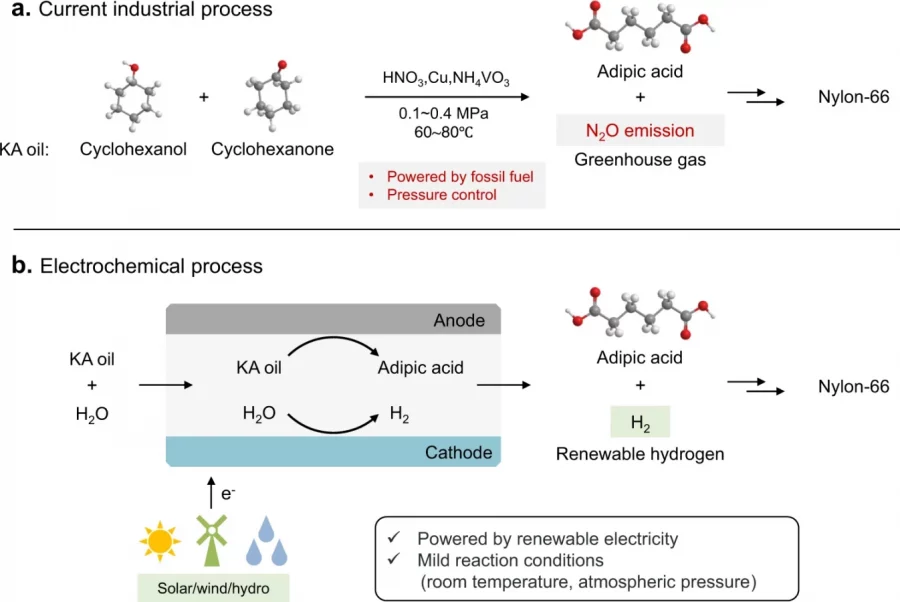
Electrocatalytic reduction of carbon dioxide (CO2ER) to produce formate is an economically viable route for CO2 upgrading. Bismuth (Bi)-based materials have been recognized as promising catalysts for this reaction but suffer from low activity that hinders their practical applications. Here, we report a cooperative catalyst of silver nanoparticles (Ag NPs) supported on a layered bismuth subcarbonate (Bi2O2CO3; BOC) nanosheet array (Ag/BOC), which achieves a 2-fold higher reaction rate in comparison to pure BOC with a high Faradaic efficiency (FE) of 98% to formate, and the FE is maintained at >85% over a wide potential window from −0.86 to −1.26 V vs RHE. Experimental results combined with theoretical results reveal that Ag accelerates the dissociation of H2O to supply reactive hydrogen species, which migrate to adjacent Bi sites to accomplish CO2 reduction with promoted activity. Moreover, we realized coproduction of formate at two poles of a homemade two-electrode flow cell by pairing the CO2ER (at the cathode) and electrooxidation of glycerol (at the anode), achieving an apparent formate FE of 130% at 2.2 V, as well as a 63% electric energy saving for formate production in comparison with the traditional CO2ER coupled with the water oxidation reaction.