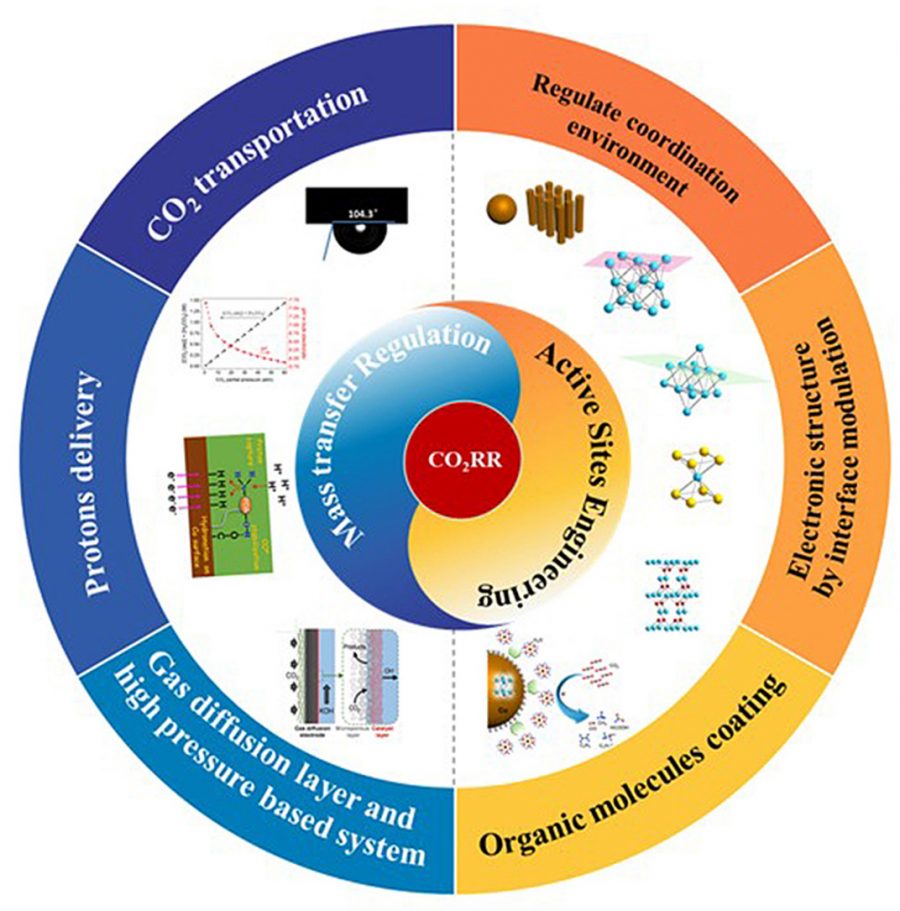
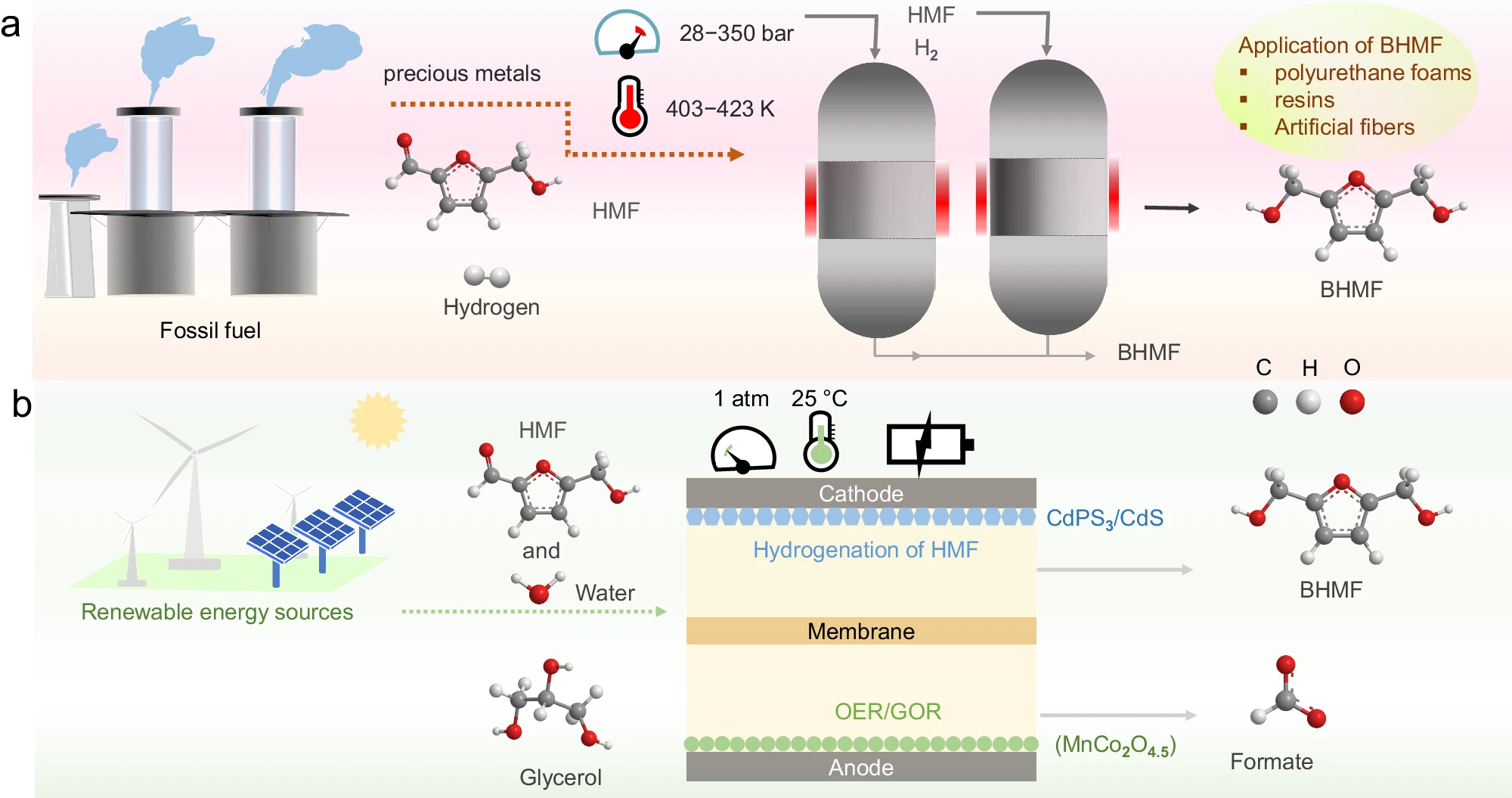
44. Electrocatalytic synthesis of adipic acid coupled with H2 production enhanced by a ligand modification strategy
Li, Z., Li, X., Zhou, H., Xu, Y., Xu, S. M., Ren, Y., Yan, Y., Yang, J., Ji, K., Li, L., Xu, M., Shao, M., Kong, X., Sun, X., Duan, H.*
Nat. Commun. 2022, 13, 5009
DOI: 10.1038/s41467-022-32769-0
Abstract
Adipic acid is an important building block of polymers, and is commercially produced by thermo-catalytic oxidation of ketone-alcohol oil (a mixture of cyclohexanol and cyclohexanone). However, this process heavily relies on the use of corrosive nitric acid while releases nitrous oxide as a potent greenhouse gas. Herein, we report an electrocatalytic strategy for the oxidation of cyclohexanone to adipic acid coupled with H2 production over a nickel hydroxide (Ni(OH)2) catalyst modified with sodium dodecyl sulfonate (SDS). The intercalated SDS facilitates the enrichment of immiscible cyclohexanone in aqueous medium, thus achieving 3.6-fold greater productivity of adipic acid and higher faradaic efficiency (FE) compared with pure Ni(OH)2 (93% versus 56%). This strategy is demonstrated effective for a variety of immiscible aldehydes and ketones in aqueous solution. Furthermore, we design a realistic two-electrode flow electrolyzer for electrooxidation of cyclohexanone coupling with H2 production, attaining adipic acid productivity of 4.7 mmol coupled with H2 productivity of 8.0 L at 0.8 A (corresponding to 30 mA cm−2) in 24 h.