53. Electrocatalytic Upcycling of Biomass and Plastic Wastes to Biodegradable Polymer Monomers and Hydrogen Fuel at High Current Densities
Yan, Y.,Zhou, H.,Xu, S. M.,Yang, J.,Hao, P.,Cai, X.,Ren, Y.,Xu, M.,Kong, X.,Shao, M.,Li, Z.*,Duan, H.*
J. Am. Chem. Soc. 2023, 145, 11, 6144–6155.
DOI: 10.1021/jacs.2c11861
Abstract
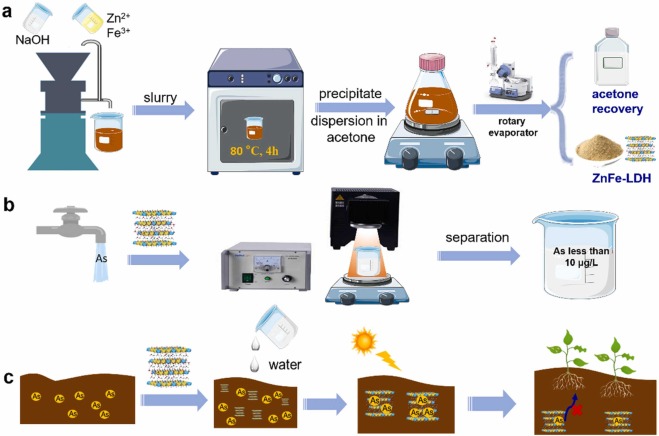
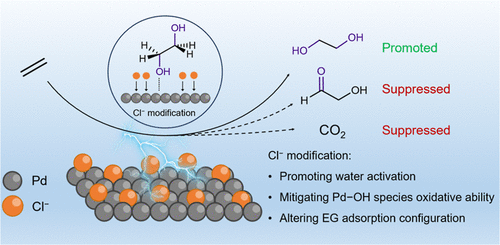
Transformation of biomass and plastic wastes to value-added chemicals and fuels is considered an upcycling process that is beneficial to resource utilization. Electrocatalysis offers a sustainable approach; however, it remains a huge challenge to increase the current density and deliver market-demanded chemicals with high selectivity. Herein, we demonstrate an electrocatalytic strategy for upcycling glycerol (from biodiesel byproduct) to lactic acid and ethylene glycol (from polyethylene terephthalate waste) to glycolic acid, with both products being as valuable monomers for biodegradable polymer production. By using a nickel hydroxide-supported gold electrocatalyst (Au/Ni(OH)2), we achieve high selectivities of lactic acid and glycolic acid (77 and 91%, respectively) with high current densities at moderate potentials (317.7 mA/cm2 at 0.95 V vs RHE and 326.2 mA/cm2 at 1.15 V vs RHE, respectively). We reveal that glycerol and ethylene glycol can be enriched at the Au/Ni(OH)2 interface through their adjacent hydroxyl groups, substantially increasing local concentrations and thus high current densities. As a proof of concept, we employed a membrane-free flow electrolyzer for upcycling triglyceride and PET bottles, attaining 11.2 g of lactic acid coupled with 9.3 L of H2 and 13.7 g of glycolic acid coupled with 9.4 L of H2, respectively, revealing the potential of coproduction of valuable chemicals and H2 fuel from wastes in a sustainable fashion.