60. Promoting Electrocatalytic Hydrogenation of 5‑Hydroxymethylfurfural over a Cooperative Ag/SnO2 Catalyst in a Wide Potential Window
Xinyue Guo, Hanchao Fu, Jiangrong Yang, Lan Luo, Hua Zhou, Ming Xu, Xianggui Kong, Mingfei Shao, Haohong Duan,* and Zhenhua Li*
ACS Catal. 2023, 13, 20, 13528–13539
DOI:10.1021/acscatal.3c03005
Abstract
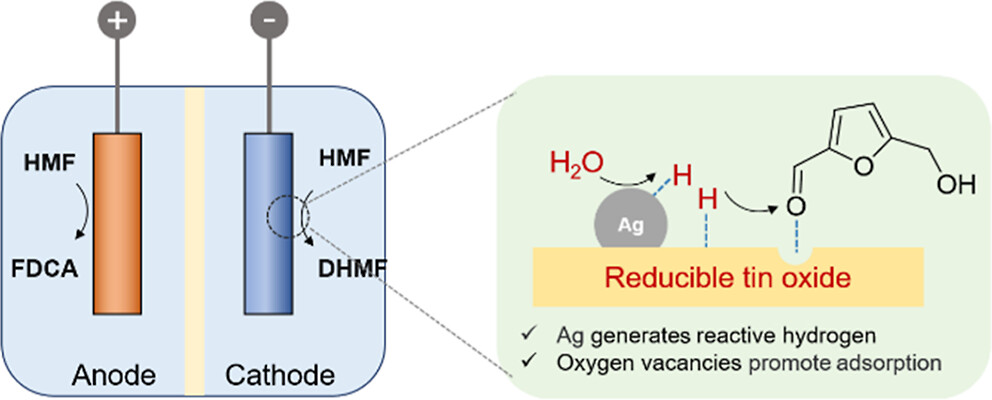
Electrochemical reduction of biomass-derived 5-hydroxymethylfurfural (HMF) to produce 2,5-dihydroxymethylfuran (DHMF) is a promising approach for biomass upgrading. The achievement of high activity and Faradaic efficiency (FE) in a wide potential window is critical for mature applications considering the significantly varied voltages supplied by different renewable energies. However, it is still challenging due to multiple reaction pathways and the competitive hydrogen evolution reaction. Herein, we synthesized a cooperative catalyst by supporting Ag nanoparticles (AgNPs) on SnO2 nanosheet arrays, which realizes the electrochemical HMF reduction to DHMF with a high FE (>95%) in a wide potential window (from −0.62 to −1.12 V vs reversible hydrogen electrode). Electrochemical and in situ measurements reveal that the AgNPs promote water splitting to generate reactive hydrogen (H*) species, which effectively react with HMF via a Langmuir–Hinshelwood mechanism. Moreover, the AgNPs accelerate the formation of oxygen vacancies on SnO2 under reaction conditions, which act as electrophilic sites to realize the selective adsorption and hydrogenation of the carbonyl bond (C═O) in HMF to yield DHMF. Finally, we designed a coupling system to simultaneously realize the electrochemical reduction and oxidation of HMF to produce DHMF and 2,5-furandicarboxylic acid, showing lower potential at the same current density than that of traditional cathodic HMF reduction coupling anodic oxygen evolution reaction, in an economical manner.