72. Electro-oxidation of 5-hydroxymethylfurfural in a low-concentrated alkaline electrolyte by enhancing hydroxyl adsorption over a single-atom supported catalyst
Xiaoxia Xia, Jingyi Xu, Xinru Yu, Jing Yang, An-Zhen Li, Kaiyue Ji, Lei Li, Min Ma, Qian Shao, Ruixiang Ge, Haohong Duan*
Sci. Bull. 2024
DOI: 10.1016/j.scib.2024.06.015

Abstract
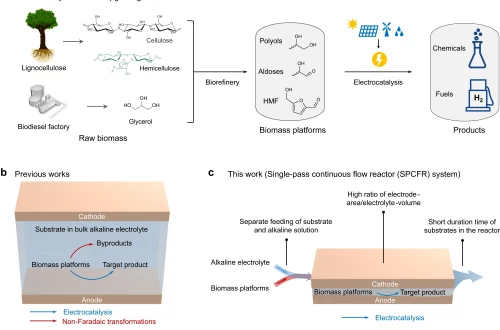
Electrocatalytic oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA), a sustainable strategy to produce bio-based plastic monomer, is always conducted in a high-concentration alkaline solution (1.0 mol L–1 KOH) for high activity. However, such high concentration of alkali poses challenges including HMF degradation and high operation costs associated with product separation. Herein, we report a single-atom-ruthenium supported on Co3O4 (Ru1-Co3O4) as a catalyst that works efficiently in a low-concentration alkaline electrolyte (0.1 mol L–1 KOH), exhibiting a low potential of 1.191 V versus a reversible hydrogen electrode to achieve 10 mA cm–2 in 0.1 mol L–1 KOH, which outperforms previous catalysts. Electrochemical studies demonstrate that single-atom-Ru significantly enhances hydroxyl (OH–) adsorption with insufficient OH– supply, thus improving HMF oxidation. To showcase the potential of Ru1-Co3O4 catalyst, we demonstrate its high efficiency in a flow reactor under industrially relevant conditions. Eventually, techno-economic analysis shows that substitution of the conventional 1.0 mol L–1 KOH with 0.1 mol L–1 KOH electrolyte may significantly reduce the minimum selling price of FDCA by 21.0%. This work demonstrates an efficient catalyst design for electrooxidation of biomass working without using strong alkaline electrolyte that may contribute to more economic biomass electro-valorization.