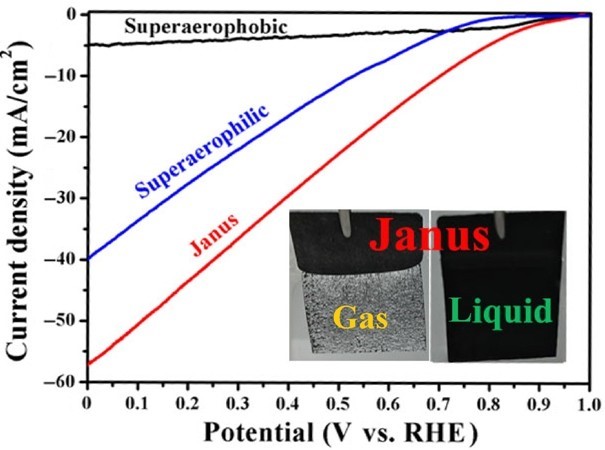
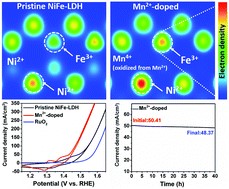
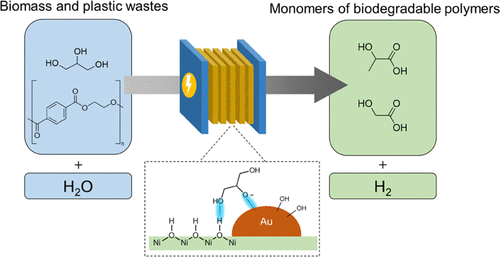
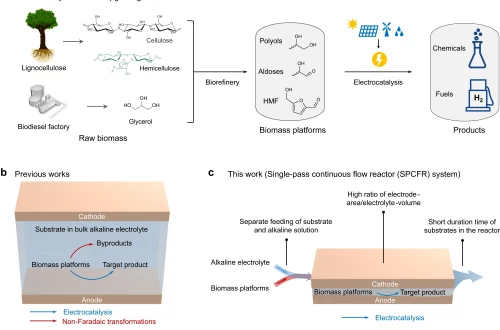
13. Highly Selective Photoreduction of CO2 with Suppressing H2 Evolution over Monolayer Layered Double Hydroxide under Irradiation above 600 nm
Tan, L., Xu, S. M., Wang, Z., Xu, Y., Wang, X., Hao, X., Bai, S., Ning, C., Wang, Y., Zhang, W., Jo, Y. K., Hwang, S. J., Cao, X., Zheng, X., Yan, H., Zhao, Y.*, Duan, H.*, Song, Y. F.*
Angew. Chem. Int. Ed. 2019, 58, 11860-11867.
DOI: 10.1002/anie.201904246
Abstract
Although progress has been made to improve photocatalytic CO2 reduction under visible light (λ>400 nm), the development of photocatalysts that can work under a longer wavelength (λ>600 nm) remains a challenge. Now, a heterogeneous photocatalyst system consisting of a ruthenium complex and a monolayer nickel-alumina layered double hydroxide (NiAl-LDH), which act as light-harvesting and catalytic units for selective photoreduction of CO2 and H2O into CH4 and CO under irradiation with λ>400 nm. By precisely tuning the irradiation wavelength, the selectivity of CH4 can be improved to 70.3 %, and the H2 evolution reaction can be completely suppressed under irradiation with λ>600 nm. The photogenerated electrons matching the energy levels of photosensitizer and m-NiAl-LDH only localized at the defect state, providing a driving force of 0.313 eV to overcome the Gibbs free energy barrier of CO2 reduction to CH4 (0.127 eV), rather than that for H2 evolution (0.425 eV).