24. An Electrocatalytic Strategy for C–C Bond Cleavage in Lignin Model Compounds and Lignin under Ambient Conditions
Ma, L., Zhou, H., Kong, X., Li, Z., Duan, H.*
ACS Sustain. Chem. Eng. 2021, 9 (4), 1932-1940.
DOI: 10.1021/acssuschemeng.0c08612
Abstract
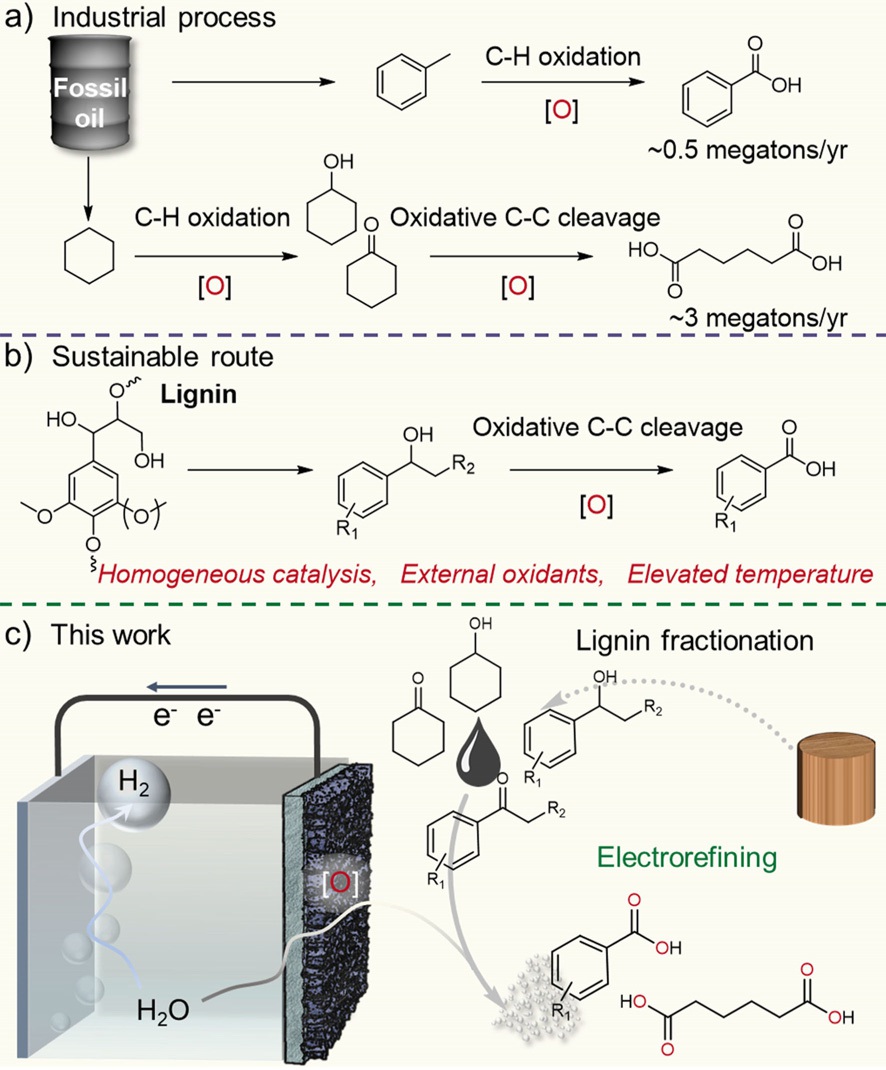
Lignin presents as a renewable aromatic resource for value-added chemical production. However, it remains challenging to cleavage C–C bonds in lignin under ambient conditions. Electrochemical oxidation as a renewable energy technology presents an attractive alternative to traditional processes. Herein, we report an efficient electrocatalytic strategy for Cα–Cβ bond cleavage of the β-O-4 linkage in lignin model compounds at room temperature with platinum as the anode and tert-butyl hydroperoxides (t-BuOOH in water, 70% aq soln) as the oxidants, exhibiting high yields for the aromatic aldehydes and phenols. This electrocatalytic strategy is demonstrated to be highly selective for the transformation of a broad selection of lignin model dimers, polymeric compound, and even real lignin into the desired aromatic aldehydes. Mechanistic studies indicate that the reaction may proceed via a Cβ radical process involving a peroxide intermediate. This work opens up opportunities in electrocatalysis to selectively break Cα–Cβ bonds and transform lignin to valuable chemicals under ambient conditions by using renewable electricity.