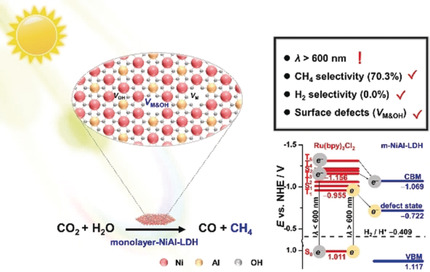
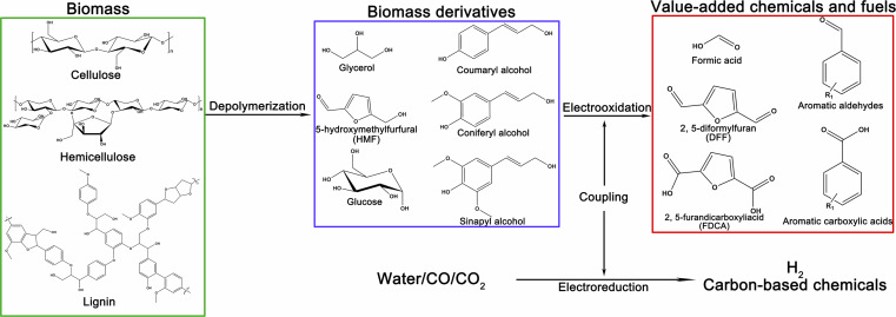
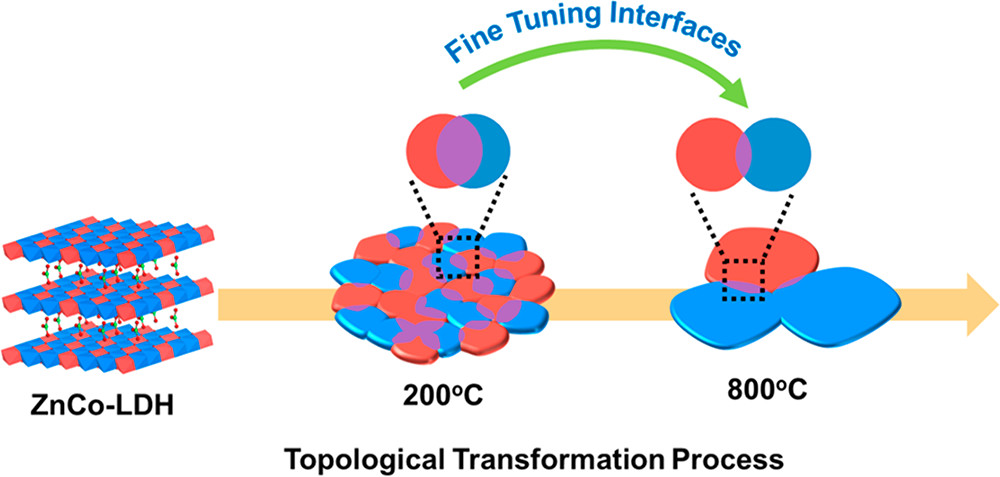
34. Selective Electrooxidation of Biomass-Derived Alcohols to Aldehydes in a Neutral Medium: Promoted Water Dissociation over a Nickel-Oxide-Supported Ruthenium Single-Atom Catalyst
Ge, R., Wang, Y., Li, Z., Xu, M., Xu, S. M., Zhou, H., Ji, K., Chen, F., Zhou, J., Duan, H.*
Angew. Chem. Int. Ed. 2022, 61 (19)
DOI: 10.1002/anie.202200211
Abstract
The biomass-derived alcohol oxidation reaction (BDAOR) holds great promise for sustainable production of chemicals. However, selective electrooxidation of alcohols to value-added aldehyde compounds is still challenging. Herein, we report the electrocatalytic BDAORs to selectively produce aldehydes using single-atom ruthenium on nickel oxide (Ru1-NiO) as a catalyst in the neutral medium. For electrooxidation of 5-hydroxymethylfurfural (HMF), Ru1-NiO exhibits a low potential of 1.283 V at 10 mA cm−2, and an optimal 2,5-diformylfuran (DFF) selectivity of 90 %. Experimental studies reveal that the neutral electrolyte plays a critical role in achieving a high aldehyde selectivity, and the single-atom Ru boosts HMF oxidation in the neutral medium by promoting water dissociation to afford OH*. Furthermore, Ru1-NiO can be extended to selective electrooxidation of a series of biomass-derived alcohols to corresponding aldehydes, which are conventionally difficult to obtain in the alkaline medium.