48. Nitrite Electroreduction to Ammonia Promoted by Molecular Carbon Dioxide with Near-unity Faradic Efficiency
Zhang, Y.,Wang, Y.,Han, L.,Wang, S.,Cui, T.,Yan, Y.,Xu, M.,Duan, H.,Kuang, Y.,Sun, X.
Angew. Chem. Int. Ed. 2023, 62 (3), e202213711
DOI: 10.1002/anie.202213711
Abstract
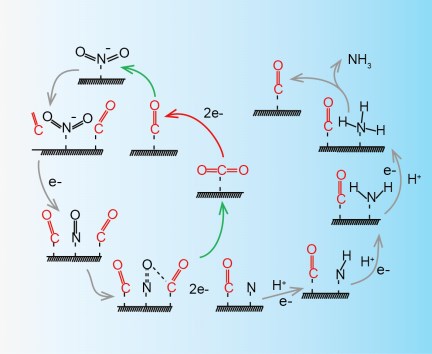
Electrochemical reduction of nitrite (NO2−) offers an energy-efficient route for ammonia (NH3) synthesis and reduction of the level of nitrite, which is one of the major pollutants in water. However, the near 100% Faradaic efficiency (FE) has yet to be achieved due to the complicated reduction route with several intermediates. Here, we report that carbon dioxide (CO2) can enhance the nitrite electroreduction to ammonia on copper nanowire (Cu NW) catalysts. In a broad potential range (-0.7 ~ -1.3 V vs. RHE), the FE of nitrite to ammonia is close to 100% with a 3.5-fold increase in activity compared to that obtained without CO2. In situ Raman spectroscopy and density functional theory (DFT) calculations indicate that CO2 acts as a catalyst to facilitate the *NO to *N step, which is the rate determining step for ammonia synthesis. The promotion effect of CO2 can be expanded to electroreduction of other nitro-compounds, such as nitrate to ammonia and nitrobenzene to aniline.