64. One-Step Electrochemical Ethylene-to-Ethylene Glycol Conversionover a Multitasking Molecular Catalyst
An-Zhen Li,# Bo-Jun Yuan,# Ming Xu, Ye Wang, Chunyu Zhang, Xiongbo Wang, Xi Wang, Jing Li,
Lirong Zheng, Bi-Jie Li,* and Haohong Duan*
J. Am. Chem. Soc. 2024, 146, 8, 5622–5633.
DOI: 10.1021/jacs.3c14381
Abstract
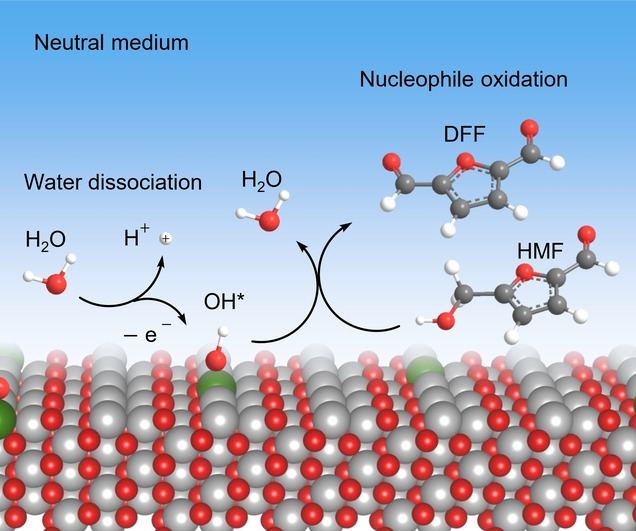
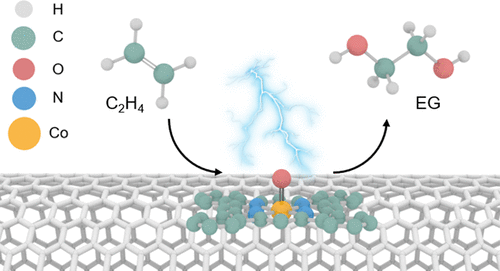
Ethylene glycol is an essential commodity chemical with high demand, which is conventionally produced via thermocatalytic oxidation of ethylene with huge fossil fuel consumption and CO2 emission. The one-step electrochemical approach offers a sustainable route but suffers from reliance on noble metal catalysts, low activity, and mediocre selectivity. Herein, we report a one-step electrochemical oxidation of ethylene to ethylene glycol over an earth-abundant metal-based molecular catalyst, a cobalt phthalocyanine supported on a carbon nanotube (CoPc/CNT). The catalyst delivers ethylene glycol with 100% selectivity and 1.78 min–1 turnover frequency at room temperature and ambient pressure, more competitive than those obtained over palladium catalysts. Experimental data demonstrate that the catalyst orchestrates multiple tasks in sequence, involving electrochemical water activation to generate high-valence Co-oxo species, ethylene epoxidation to afford an ethylene oxide intermediate via oxygen transfer, and eventually ring-opening of ethylene oxide to ethylene glycol facilitated by in situ formed Lewis acid site. This work offers a great opportunity for commodity chemicals synthesis based on a one-step, earth-abundant metal-catalyzed, and renewable electricity-driven route.