67. Promoting Electrocatalytic Semihydrogenation of Alkynols to Alkenols over a Bimetallic CuAu Alloy Catalyst
Qinghui Ren, Leilei Hao, Jiangrong Yang, Mengyu Lv, Hua Zhou, Zhenhua Li,* Haohong Duan,* and Mingfei Shao*
ACS Catal. 2024, 14, 8, 5675–5684
DOI:10.1021/acscatal.3c05928
Abstract
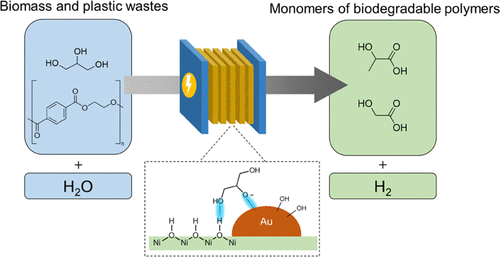
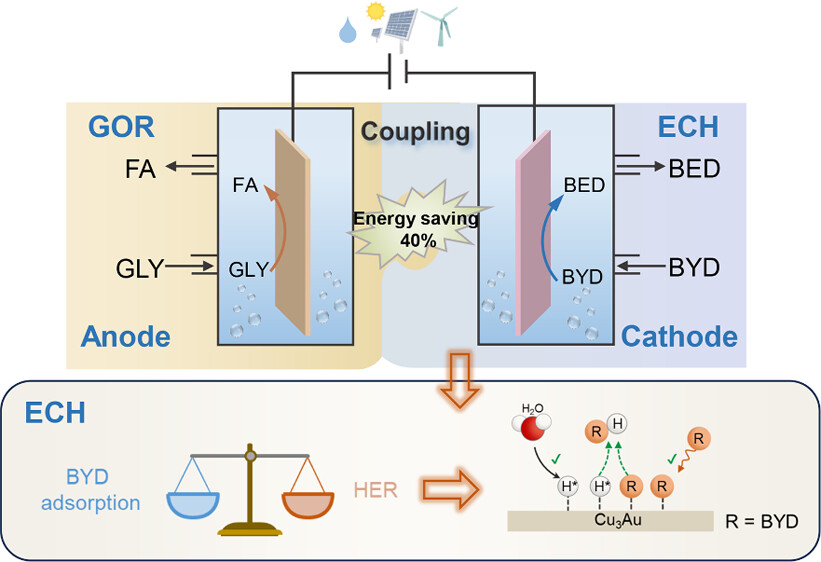
Electrocatalytic semihydrogenation of alkynols to alkenols under ambient conditions using H2O as a hydrogen source is highly attractive in synthetic chemistry. However, it is still challenging to achieve a high Faradaic efficiency (FE) in a wide potential window. Herein, we reported a bimetallic Cu3Au alloy as an efficient catalyst for electrocatalytic semihydrogenation of alkynols to alkenols. Specifically, during semihydrogenation of 2-butyne-1,4-diol (BYD) to 2-butene-1,4-diol (BED), the Cu3Au catalyst achieves 12.6-fold greater reaction rate and higher FE compared with pure Cu (99 vs 63%). Moreover, the Cu3Au
maintains >96% FEs in a wide potential window from −0.19 to −0.59 V vs RHE. We demonstrate that the competitive adsorptions of reactive hydrogen (H*) and BYD greatly influence the semihydrogenation processes. The presence of Au in Cu3Au facilitates H* formation and reduces BYD adsorption on Cu, thus enhancing the BYD hydrogenation performance. The Cu3Au catalyst affords a broad substrate scope from alkynols to aromatic alkynes, producing the corresponding alkenes in good selectivities. Finally, we coupled BYD semihydrogenation with glycerol oxidation to replace oxygen evolution reaction in a two-electrode system, showing 40% energy saving at 200 mA for BED production and coproduction of valuable formate at the anode, demonstrating an economical manner.