57. An Anion Leaching Strategy towards Metal Oxyhydroxides Synthesis for Electrocatalytic Oxidation of Glycerol
Ye Wang , Ruixiang Ge , Xiang Liu , Jing Li , Haohong Duan*
Acta Phys. -Chim. Sin. 2024, 40, 2307019
DOI: 10.3866/PKU.WHXB202307019
Abstract
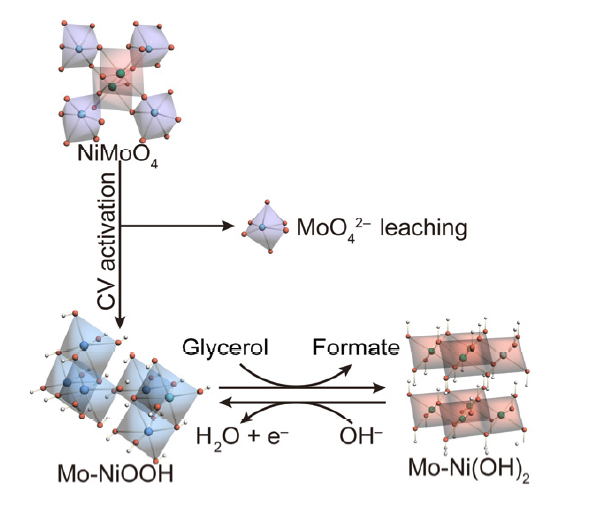
Nucleophile oxidation reaction (NOR) is emerging as a significant approach for the sustainable production of value-added chemicals. Among the various types, electrocatalytic glycerol oxidation reaction (GOR) stands out as a crucial method for producing C1 to C3 chemicals including formic acid (FA). Nonnoble- metal-based (oxy)hydroxides have found extensive use in GOR, yet achieving industrially-demanded current densities (> 300 mA·cm−2) at moderate potentials remains a challenge. It is well documented that GOR catalyzed by (oxy)hydroxides follows an indirect oxidation mechanism. Specifically, the nucleophile, glycerol, undergoes oxidation by the electrogenerated oxyhydroxides with electrophilic adsorption oxygen. Therefore, comprehending the evolution of the electrocatalyst in GOR is critically important. In this paper, we have developed molybdenum-doped nickel oxyhydroxides (Mo-NiOOH) through cyclic voltammetry (CV) activation of nickel molybdate (NiMoO4). We demonstrated that Mo species leach from NiMoO4, and the resulting Mo-NiOOH retains the nanosheet array morphology
of NiMoO4. We subjected the freshly prepared Mo-NiOOH to systematic characterizations employing techniques such as scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS) mapping, Raman spectroscopy, inductively coupled plasma-mass spectrometry (ICP-MS), and X-ray photoelectron spectroscopy (XPS). The above structural characterizations confirm that Mo-NiOOH inherits the nanosheet array morphology of the NiMoO4 precursor with reduced Mo content, thereby indicating the phase reconstruction from oxides to oxyhydroxides post CV activation. Furthermore, the Ni3+/Ni2+ ratio in Mo-NiOOH surpasses that in NiOOH derived from CV activation of Ni(OH)2. Mo-NiOOH exhibits elevated electrochemically active surface areas (ECSAs) and a higher Ni3+/Ni2+ ratio compared to NiOOH obtained through CV activation of Ni(OH)2, facilitating the Mo-NiOOH exhibits higher ratio of Ni3+/Ni2+, higher electrochemically active surface areas (ECSAs) than NiOOH, and facilitated oxidation of Ni2+ to Ni3+. Consequently, Mo-NiOOH requires a lower applied potential than NiOOH (1.51 V versus 1.84 V vs. reversible hydrogen electrode (RHE)) to achieve a high current density (400 mA·cm−2). Additionally, Mo-NiOOH demonstrates higher Faradaic efficiency towards formate (FEformate) in contrast to NiOOH (84.7% versus 59.6%), indicating enhanced carbon-carbon (C―C) bond cleavage due to Mo doping. Multi-potential step (STEP) experiments indicate that GOR catalyzed by NiOOH and Mo-NiOOH follows a similar indirect oxidation mechanism mediated by oxyhydroxides. Operando electrochemical impedance spectroscopy (EIS) and in situ Raman spectroscopy confirmed that Mo doping in NiOOH accelerates GOR kinetics and the oxidation of Ni2+ to Ni3+, contributing to the higher activity and formate selectivity of Mo-NiOOH than NiOOH. The strategy of surface modulation of oxyhydroxides through leaching of soluble anions offers guidelines for the rational design of high-performance NOR
electrocatalysts.