12. Introducing Fe2+ into Nickel–Iron Layered Double Hydroxide: Local Structure Modulated Water Oxidation Activity
Cai, Z., Zhou, D., Wang, M., Bak, S. M., Wu, Y., Wu, Z., Tian, Y., Xiong, X., Li, Y., Liu, W., Siahrostami, S., Kuang, Y., Yang, X. Q., Duan, H.*, Feng, Z.*, Wang, H.*, Sun, X.*
Angew. Chem. Int. Ed. 2018, 57, 9392-9396.
DOI: 10.1002/anie.201804881
Abstract
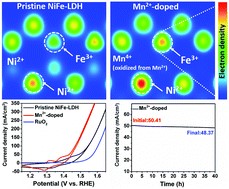
Exploring materials with regulated local structures and understanding how the atomic motifs govern the reactivity and durability of catalysts are a critical challenge for designing advanced catalysts. Herein we report the tuning of the local atomic structure of nickel–iron layered double hydroxides (NiFe-LDHs) by partially substituting Ni2+ with Fe2+ to introduce Fe-O-Fe moieties. These Fe2+-containing NiFe-LDHs exhibit enhanced oxygen evolution reaction (OER) activity with an ultralow overpotential of 195 mV at the current density of 10 mA cm−2, which is among the best OER catalytic performance to date. In-situ X-ray absorption, Raman, and electrochemical analysis jointly reveal that the Fe-O-Fe motifs could stabilize high-valent metal sites at low overpotentials, thereby enhancing the OER activity. These results reveal the importance of tuning the local atomic structure for designing high efficiency electrocatalysts.